Microsphere | Looking beneath the surface
Views on the world of food and beverage research, nanotechnology,
tricky chemical analysis and some thoughts on beer!
tricky chemical analysis and some thoughts on beer!
|
What comes to mind when you think about yeast, trusty old Saccharomyces cerevisiae? Brewers and distillers are well acquainted with yeast as the agent of fermentation. The extended use of yeast outside of its traditional areas of ethanol production and bread making has moved from the simple use of yeast extract to produce Marmite® into the areas of biomanufacturing, nutraceuticals and personal care products. The nutritional content of yeast has historically focused on yeast as a source of single cell protein and for its B vitamin content. In more recent years the market for dietary supplements has exploded and the opportunity of marketing brewers’ yeast as such has increased. Yeast based products are being proposed for use in cosmetics formulations by bulk yeast suppliers perhaps more normally associated with brewing and baking. Transgenic yeast are being exploited to synthesize secondary metabolites for pharmaceutical intermediates and mutants have been selected for the over production of fragrance molecules such as 2-phenylethanol. Progress has been made in the exploitation of yeast as an inert carrier of small molecules. Commercial products consisting of mineral-enriched yeast have been available for some time. Zinc-enriched yeast cells are being used in the brewing industry and selenium-enriched products have been approved for use as nutrient supplements in food. The potential for encapsulating other chemicals inside yeast is less widely known. Materials that can be encapsulated in spent yeast include flavours, pesticides and active pharmaceutical ingredients (APIs). Microencapsulation For many years microencapsulation has been used as a tool to convert liquids to solids to improve handling. The products formed as a result of encapsulation can be stored as dry powders or granules thus volatile and flammable flavours can be handled more easily and shipped and stored with few restrictions. Microencapsulation can be used in targeted delivery to improve the impact of active ingredients, to control or delay release for masking taste and odour; to improve process stability; to protect materials sensitive to UV, heat, oxidation and high or low pH; for the isolation of reactive components and to stabilize starter cultures, probiotics and enzymes. Materials typically used include natural or synthetic polymers, gums, starches and lipids. Coatings and particles are formed using a range of methods including fluid bed coating, coacervation, spray drying and spray chilling. Typically microcapsules are formed in situ although in some cases absorption is also used to stabilize molecules, for example with cyclodextrin molecular inclusion technology or when flavours are spray dried onto maltodextrin and gums or resins. Yeast is a natural alternative to other encapsulation materials and offers unique performance benefits. A number of encapsulation technologies using yeast have been developed and refined. The use of yeast cells as biocapsules was first considered in the 1970s when Serozym Laboratories discovered that cells (Saccharomyces cerevisiae), treated with a plasmolyzer, could be used to absorb water soluble substances for use in medical, cosmetic and food products. Yeast, widely available in large volumes as a by-product of fermentation processes, was identified as a cost effective material for use in these global industries. Yeast has a complex structure; it comprises a dense absorbent cytoplasm rich in organelles, lipid membranes and lipid droplets, protected by a 7-12 nm lipid bilayer and heterogeneous cell wall. The surrounding cell wall is typically up to 200 nm thick, rich in beta-glucan, mannoproteins and chitin and provides robust protection to the cell contents as can be seen in Figure 1. In 1977 the US company Swift and Co. utilized yeast strains that accumulated lipid to a high internal concentration (>40% w/w) when grown on specific nitrogen rich media. These yeast strains were particularly effective in absorbing fat soluble dyes, vitamins and drugs which dissolved in the free lipid in the cell cytoplasm. The technology, based on an aqueous mixing process, was developed further by UK based company AD2 Ltd. This was taken to a commercial scale in Europe and the USA, initially as a flavour delivery encapsulation process, by companies including Micap plc and Corn Products Corporation. In some cases, materials can be stabilized in yeast at levels as high as 45% by weight of the powdered or granulated end product. There is potential for using waste brewery yeast or spent yeast from ethanol biofuel production with these yeast encapsulation technologies, primarily for use in food systems to improve flavour delivery, flavour stability, appearance and nutritional value. There is also potential for the encapsulation of materials for non-food applications such as pesticides, herbicides, antibacterials, antifungals and essential oils. Traditionally excess or waste brewery yeast was sold into the malt whisky distilling industry in the UK to supplement distillers yeast and improve the flocculation properties in fermentation vessels. This was summarised in a 1985 guidance document from the Institute of Brewing and the Allied Brewery Traders’ Association. One requirement of this market is the consistent supply of viable yeast. The sale of spent Brewer’s yeast into the food processing industry has been a source of income for brewers in the past, however, the demands for higher quality ingredients at lower cost combined with the increasing costs of supply has reduced the opportunities for the brewer in this area. The supply of segregated and suitably packaged products is required to successfully penetrate the food and feed markets. Slurry, cake, dry powder and granulated products are typically available for supply. However, for food, pet food and many animal feed applications debittering to remove hop components is a prerequisite to improve palatability and may be undertaken by third party processors. Spent yeast is typically deactivated during processing and live yeast is not required for the absorption of active ingredients to take place. The details of yeast based encapsulation using an aqueous mixing process have been described in new patents and in the scientific literature since the late 1980s. Effective aqueous based encapsulation using yeast depends on the active ingredients to have a small degree of water solubility to facilitate partitioning of the active ingredient into the yeast inner matrix. Biocapsules typically encapsulate well those chemicals with partition coefficients around Log Po/w 2.0. A typical encapsulation process flow using yeast as an inert carrier is illustrated in Figure 2. The process can take up to 24 hours for the yeast to absorb the maximum quantity of material. 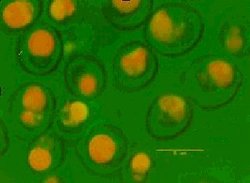 Figure 3: Sage oil encapsulated in cells of Saccharomyces cerevisiae visualized using confocal microscopy Figure 3: Sage oil encapsulated in cells of Saccharomyces cerevisiae visualized using confocal microscopy Each individual cell acts as a sink for hydrophobic molecules and can accumulate material to a high concentration. The presence of droplets found within the 4-7 micron yeast capsule, has been visualized using confocal microscopy and Figure 3 shows essential oil droplets, coloured orange, that have accumulated to around 400 g/kg dry wt.
A high degree of membrane and cell structural integrity remains following the encapsulation process. The intact cell wall and membrane can be clearly seen in Figure 5 following two hours of mixing with Tea Tree oil. Droplets a few tens of nanometers form initially and are distributed throughout the cytoplasm. These typically coalesce later in the process. Some droplets can be observed on the cell wall surface prior to being absorbed into the yeast. The rate of absorption by spent yeast is not the same for all active ingredients and it is primarily the permeability of the yeast cell wall that acts as the selective barrier rather than the cell membrane. Figure 6 illustrates the relative potential for small molecules to be absorbed and stabilized within yeast cells. Molecules with low partition coefficients readily pass into the cells but will not accumulate and pass back out into the water during mixing. Once encapsulated, poorly water soluble materials can be dispersed more readily in aqueous systems without the aid of solvents or surfactants. The evaporation of volatile materials from the processed dry powder can be minimized thus improving shelf life performance and process stability in applications such as baking. Molecules with low partition coefficients readily pass into the cells but will not accumulate and pass back out into the water during mixing. Once encapsulated, poorly water soluble materials can be dispersed more readily in aqueous systems without the aid of solvents or surfactants. The evaporation of volatile materials from the processed dry powder can be minimized thus improving shelf life performance and process stability in applications such as baking. Delivering the goods The release of components from traditional microcapsules can be achieved using physical pressure. For example, in carbonless copy paper when writing the pressure of the pen tip on the capsule containing paper breaks open the microcapsules. The encapsulated dye is released into an acid clay matrix which produces a colour change in the dye. Alternatively, in various applications, soluble starches dissolve or fat coatings melt releasing their payload. Yeast cells are robust and are resistant to physical damage, shear pressure, heat and chemical stresses. For example, dried powder formulations of food flavours can retain flavours even at 200 C. However, on the addition of water, dried yeast biocapsules are primed to release absorbed hydrophobic materials such as volatile flavour components. The release is facilitated, in most cases, by diffusion down a concentration gradient from source to sink. This happens in the mouth when food is eaten and food flavours are released into the saliva and evaporate into the buccal cavity. Future developments Screening for pharmaceutical ingredients (APIs) from combinatorial chemistry strategies can produce 40% of targets that are poorly water-soluble. These difficult to formulate molecules are more frequently being subjected to nanotechnology approaches. There are advantages in stabilising APIs as very fine dispersions and yeast offers an alternative approach for stabilising nanodispersions by housing them within a biocapsule. Thus yeast could be presented as a vehicle for the oral and buccal delivery of these difficult to formulate APIs. One feature of the established process is its restriction to small hydrophobic molecules and principally molecules with octanol/water partition coefficients below Log P 4.0 and molecular weights below 600 Da. It works well, for example with volatile food flavours, some small drug molecules and a limited range of crop protection active ingredients. Recent developments enable the encapsulation of large, to up to 5000 Da, and very hydrophobic compounds in biocapsules for the first time. Potential candidates include active ingredients such as insecticides (e.g. deltramethrin, ivermectin), fungicides (e.g. carboxin, epoxiconazole), molluscicides, (e.g. fentin, methiocarb), nematicides (e.g. carbofuran), rodenticides (e.g. brodifacoum, norbormide), herbicides (e.g. oxasulfuron) and poorly soluble active pharmaceutical ingredients (Class II and Class IV drugs) (e.g. fenofibrate and ketoconazole). This new approach differs from previous biocapsules processes in one key area: the removal of water from the encapsulation process. Water is replaced by a solvent such as dimethylsulphoxide in which many difficult to formulate active ingredients are readily soluble. Dilution with water after the encapsulation process can be used to stabilize the hydrophobic molecules within the cells. Following a period of mixing, the product can be spray dried; alternatively, an aqueous dispersion can be used directly. This moves us into areas where patent licensing is required, costs of processing are high and where there are demands for dedicated or GMP yeast manufacturing. This is a long way from finding an alternative use of spent brewery yeast. Innovation using yeast biocapsules The abundance of prior art for water based bioencapsulation systems has restricted commercial exploitation. Patents filed independently in Europe and Japan resulted in a challenging market segmentation situation. This is compounded by application based patent filings in areas such as smoking cessation products; tobacco products; homecare products; drug delivery; cosmetics; pesticides and flavouring baked goods. Many patents, particularly on the process side, have now lapsed or are due to lapse opening up the potential for commercial exploitation by the brewing and allied industries as an alternative revenue stream. In conclusion, using spent yeast as an inert carrier may in some circumstances add value in providing a complex matrix material in which active ingredients can be stabilized as a reservoir or depot. Spent yeast is a potential vehicle to provide a sustained release profile in situ for food flavours or perhaps in the future for crop protection products. The technology is being commercially exploited already so there is clearly life in the old dog yet. References and further reading AD2 Ltd. European patent (1987), EP 0242135.
Bishop J., Nelson G., Lamb J. (1998). Microencapsulation in Yeast. Journal of Microencapsulation, 15, (6 ), 761-773. Dardelle, G., Normand, V., Steenhoudt, M., Bouquerand, P.E., Chevalier, M., Baumgartner, P., 2007. Flavour-encapsulation and flavour-release performances of a commercial yeast-based delivery system. Food Hydrocolloids. 21, 953–960. Dunlop Ltd 1986 UK Patent GB2162147 Duckham S. C., Burgess, A., Hinds, L. and Echlin, P. (2003) Microencapsulation in yeast cells - Structure and function: A cryo SEM approach. In: Proceedings of the 14th International Symposium on Microencapsulation, September 4-6, 2003, Singapore. Kilcher, G., Delneri D., Duckham C. and Tirelli N. (2008) Probing (macro)molecular transport through cell walls. Faraday Discuss., 139, 199–212 Mattanovich, D., Sauer, M. and Gasse, B. (2014). Yeast biotechnology: teaching the old dog new tricks. Microbial Cell Factories. 13 (1) :34 Nelson G., Duckham S.C., Crothers M.E.D. (2006). Microencapsulation in yeast cells and Application in Drug Delivery. Polymeric Drug Delivery, ACS Symposium Series 923, Chapter 19, 268-281. Normand V., Dardelle G., Bouquerand P-E, Nicolas L., Johnston D. J. (2005). Flavour Encapsulation in Yeasts: Limonene used as a Model System for Characterization of the Release Mechanism. Journal of Agricultural & Food Chemistry, 53, 7532-7542.
0 Comments
|
Craig is a consultant, a flavour enthusiast and an unapologetic analytical chemistry geek.Archives
June 2017
Categories
All
|